Flammability limit
Flammability limits, also called flammable limits, give the proportion of combustible gases in a mixture, between which limits this mixture is flammable. Gas mixtures consisting of combustible, oxidizing, and inert gases are only flammable under certain conditions. The lower flammable limit (LFL) describes the leanest mixture that still sustains a flame, i.e. the mixture with the smallest fraction of combustible gas, while the upper flammable limit (UFL) gives the richest flammable mixture.
There is a quantitative difference between flammability limits and explosive limits. In an explosive mixture the fuel oxidizer mixture is closer to stoichiometric proportion. This difference has no practical application in safety engineering as the flammable vapor cloud is turbulent and the exact mixture of fuel and oxidizer varies greatly. Therefore, many references use the term flammability limit(LFL, UFL) and explosive limit (LEL, UEL) interchangeably.
Attaining the perfect combustible or explosive mixture between a fuel and air is important in internal combustion engines, for example in gasoline or diesel engines.
A deflagration is a propagation of a combustion zone at a velocity less than the speed of sound in the unreacted medium. A detonation is a propagation of a combustion zone at a velocity greater than the speed of sound in the unreacted medium. An explosion is the bursting or rupture of an enclosure or container due to the development of internal pressure from a deflagration or detonation as defined in NFPA 69.
Contents |
Limits
Lower Explosive Limit
Lower Explosive Limit (LEL): The lowest concentration (percentage) of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source (arc, flame, heat). At a concentration in air below the LEL there is not enough fuel to continue an explosion. Concentrations lower than the LEL are "too lean" to explode but may still deflagrate. Methane gas has a LEL of 4.4% (at 138 degrees C) by volume, meaning 4.4% of the total volume of the air consists of methane. At 20 degrees C the LEL is 5.1 % by volume. If the atmosphere has less than 5.1% methane, an explosion cannot occur even if a source of ignition is present. When methane (CH4) concentration reaches 5.1% an explosion can occur if there is an ignition source. LEL concentrations vary greatly between combustible gases.
Percentage reading on combustible air monitors should not be confused with the LEL concentrations. Explosimeters designed and calibrated to a specific gas may show the relative concentration of the atmosphere to the LEL - the LEL being 100%. A 5% displayed LEL reading for methane, for example, would be equivalent to 5.1% multiplied by 5%, or approximately 0.25% methane by volume at 20 degrees C. Control of the explosion hazard is usually achieved by sufficient natural or mechanical ventilation, to limit the concentration of flammable gases or vapors to a maximum level of 25% of their Lower Explosive or Flammable Limit.
Upper Explosive Limit
Upper Explosive Limit (UEL): Highest concentration (percentage) of a gas or a vapor in air capable of producing a flash of fire in presence of an ignition source (arc, flame, heat). Concentration higher than UFL or UEL are "too rich" to burn.
Influence of temperature, pressure and composition
Flammability limits of mixtures of several combustible gases can be calculated using Le Chatelier's mixing rule for combustible volume fractions xi:
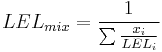
and similar for UEL.
Temperature, pressure, and the concentration of the oxidizer also influences flammability limits. Higher temperature results in lower LFL and higher UFL, while greater pressure increases both values. The effect of pressure is very small at pressures below 10 millibar and difficult to predict, since it has only been studied in internal combustion engines with a turbocharger.
Oxygen enriched atmospheres lower the LFL and increase the UFL. An atmosphere devoid of an oxidizer is neither flammable or explosive regardless of the fuel gas concentration. Increasing the fraction of inert gases in an air mixture raises the LFL and decreases the UFL.
Controlling explosive atmospheres
Controlling gas and vapor concentrations outside the explosive limits is a major consideration in occupational safety and health. Methods used to control the concentration of a potentially explosive gas or vapor include use of sweep gas, an unreactive gas such as nitrogen or argon to dilute the explosive gas before coming in contact with air. Use of scrubbers or adsorption resins to remove explosive gases before release are also common. Gases can also be maintained safely at concentrations above the UEL, although a breach in the storage container can lead to explosive conditions or intense fires.
Dusts
Dusts also have upper and lower explosion limits, though the upper limits are hard to measure and of little practical importance. Lower explosive limits for many organic materials are in the range of 10–50 g/m³, which is much higher than the limits set for health reasons, as is the case for the LEL of many gases and vapours. Dust clouds of this concentration are hard to see through for more than a short distance, and normally only exist inside process equipment.
Explosion limits also depend on the particle size of the dust involved, and are not intrinsic properties of the material. In addition, a concentration above the LEL can be created suddenly from settled dust accumulations, so management by routine monitoring, as is done with gases and vapours, is of no value. The preferred method of managing combustible dust is by preventing accumulations of settled dust through process enclosure, ventilation, and surface cleaning. However, lower explosion limits may be relevant to plant design.
Examples
The flammable/explosive limits of some gases and vapors are given below. Concentrations are given in percent by volume of air.
- Class IA liquids (Flash Point less than 73°F (22.8°C); Boiling Point less than 100°F (37.8°C) are NFPA 704 Flammability Rating 4
- Classes IB (Flash Point less than 73°F (22.8°C); Boiling Point equal to or greater than 100°F (37.8°C)) and IC liquids (Flash Point equal to or greater than 73°F (22.8°C), but less than 100°F (37.8°C)) are NFPA 704 Flammability Rating 3
- Classes II (Flash Point equal to or greater than 100°F (37.8°C), but less than 140°F) and IIIA liquids (Flash Point equal to or greater than 140°F (60°C), but less than 200°F (93.3°C)) are NFPA 704 Flammability Rating 2
- Class IIIB liquids (Flash Point equal to or greater than 200°F (93.3°C) are NFPA 704 Flammability Rating 1
| Substance | LFL/LEL in %
by volume of air |
UFL/UEL in %
by volume of air |
NFPA Class | Flash point | Minimum Ignition Energy in mJ expressed as percent by volume in air (Note, for many chemicals it |
Autoignition Temperature |
|---|---|---|---|---|---|---|
| Acetaldehyde | 4.0 | 57.0 | IA | -39°C | 0.37 | 175°C |
| Acetic acid (glacial) | 4 | 19.9 | II | 39°C to 43°C | 463°C | |
| Acetic anhydride | II | 54°C | ||||
| Acetone | 2.6 - 3 | 12.8 - 13 | IB | -17°C | 1.15 @ 4.5% | 465°C, 485°C[2] |
| Acetonitrile | IB | 2°C | 524°C | |||
| Acetyl chloride | 7.3 | 19 | IB | 5°C | 390°C | |
| Acetylene | 2.5 | 82 | IA | -18°C | 0.017 @ 8.5% (in pure oxygen 0.0002 @ 40%) | 305°C |
| Acrolein | 2.8 | 31 | IB | -26°C | 0.13 | |
| Acrylonitrile | 3.0 | 17.0 | IB | 0°C | 0.16 @ 9.0% | |
| Allyl chloride | 2.9 | 11.1 | IB | -32 °C | 0.77 | |
| Ammonia | 15 | 28 | IIIB | 11°C | 680 | 651°C |
| Arsine | 4.5 - 5.1[3] | 78 | IA | Flammable gas | ||
| Benzene | 1.2 | 7.8 | IB | -11°C | 0.2 @ 4.7% | 560°C |
| 1,3-Butadiene | 2.0 | 12 | IA | -85°C | 0.13 @ 5.2% | |
| Butane, n-Butane | 1.6 | 8.4 | IA | -60°C | 0.25 @ 4.7% | 420 - 500°C |
| n-Butyl acetate, Butyl acetate | 1 - 1.7[4] | 8 - 15 | IB | 24°C | 370°C | |
| Butyl alcohol, Butanol | 1 | 11 | IC | 29°C | ||
| n-Butanol | 1.4[5] | 11.2 | IC | 35°C | 340°C | |
| n-Butyl chloride, 1-chlorobutane | 1.8 | 10.1 | IB | -6°C | 1.24 | |
| n-Butyl mercaptan | 1.4[6] | 10.2 | IB | 2°C | 225°C | |
| Butyl methyl ketone, 2-Hexanone | 1[7] | 8 | IC | 25°C | 423°C | |
| Butylene, 1-Butylene, 1-Butene | 1.98[8] | 9.65 | IA | -80°C | ||
| Carbon disulfide | 1.0 | 50.0 | IB | -30°C | 0.009 @ 7.8% | 90°C |
| Carbon Monoxide | 12[9] | 75 | IA | -191°C Flammable gas | 609°C | |
| Chlorine monoxide | IA | Flammable gas | ||||
| 1-Chloro-1,1-difluoroethane | 6.2 | 17.9 | IA | -65°C Flammable Gas | ||
| Cyanogen | 6.0 - 6.6[10] | 32 - 42.6 | IA | Flammable gas | ||
| Cyclobutane | 1.8 | 11.1 | IA | -63.9°C[11] | 426.7°C | |
| Cyclohexane | 1.3 | 7.8 - 8 | IB | -18°C - -20°C[12] | 0.22 @ 3.8% | 245°C |
| Cyclohexanol | 1 | 9 | IIIA | 68°C | 300°C | |
| Cyclohexanone | 1 - 1.1 | 9 - 9.4 | II | 43.9 - 44°C | 420°C[13] | |
| Cyclopentadiene[14] | IB | 0°C | 0.67 | 640°C | ||
| Cyclopentane | 1.5 - 2 | 9.4 | IB | - 37 to -38.9°C[15][16] | 0.54 | 361°C |
| Cyclopropane | 2.4 | 10.4 | IA | -94.4°C[17] | 0.17 @ 6.3% | 498°C |
| Decane | 0.8 | 5.4 | II | 46.1°C | 210°C | |
| Diborane | 0.8 | 88 | IA | -90°C Flammable gas[18] | 38°C | |
| o-Dichlorobenzene, 1,2-Dichlorobenzene | 2[19] | 9 | IIIA | 65°C | 648°C | |
| 1,1-Dichloroethane | 6 | 11 | IB | 14°C | ||
| 1,2-Dichloroethane | 6 | 16 | IB | 13°C | 413°C | |
| 1,1-Dichloroethene | 6.5 | 15.5 | IA | -10°C Flammable gas | ||
| Dichlorofluoromethane | 54.7 | Non flammable,[20] -36.1°C[21] | 552°C | |||
| Dichloromethane, Methylene chloride | 16 | 66 | Non flammable | |||
| Dichlorosilane | 4 - 4.7 | 96 | IA | -28 °C | 0.015 | |
| Diesel fuel | 0.6 | 7.5 | IIIA | >62°C (143°F) | 210°C | |
| Diethanolamine | 2 | 13 | IB | 169°C | ||
| Diethylamine | 1.8 | 10.1 | IB | -23°C to -26°C | 312°C | |
| Diethyl disulfide | 1.2 | II | 38.9°C[22] | |||
| Diethyl ether | 1.9 - 2 | 36 - 48 | IA | -45°C | 0.19 @ 5.1% | 160 - 170°C |
| Diethyl sulfide | IB | -10°C[23] | ||||
| 1,1-Difluoroethane | 3.7 | 18 | IA | -81.1°C[24] | ||
| 1,1-Difluoroethylene | 5.5 | 21.3 | -126.1°C[25] | |||
| Diisobutyl ketone | 1 | 6 | 49°C | |||
| Diisopropyl ether | 1 | 21 | IB | -28°C | ||
| Dimethylamine | 2.8 | 14.4 | IA | Flammable gas | ||
| 1,1-Dimethylhydrazine | IB | |||||
| Dimethyl sulfide | IA | -49°C | ||||
| Dimethyl sulfoxide | 2.6 - 3 | 42 | IIIB | 88 - 95°C | 215°C | |
| 1,4-Dioxane | 2 | 22 | IB | 12°C | ||
| Epichlorohydrin | 4 | 21 | 31°C | |||
| Ethane | 3[26] | 12 - 12.4 | IA | Flammable gas -135 °C | 515°C | |
| Ethanol, Ethyl Alcohol | 3 - 3.3 | 19 | IB | 12.8°C (55°F) | 365°C | |
| 2-Ethoxyethanol | 3 | 18 | 43°C | |||
| 2-Ethoxyethyl acetate | 2 | 8 | 56°C | |||
| Ethyl acetate | 2 | 12 | IA | -4°C | 460°C | |
| Ethylamine | 3.5 | 14 | IA | -17 °C | ||
| Ethylbenzene | 1.0 | 7.1 | 15-20 °C | |||
| Ethylene | 2.7 | 36 | IA | 0.07 | 490°C | |
| Ethylene glycol | 3 | 22 | 111°C | |||
| Ethylene oxide | 3 | 100 | IA | −20 °C | ||
| Ethyl Chloride | 3.8[27] | 15.4 | IA | −50°C | ||
| Ethyl Mercaptan | IA | |||||
| Fuel oil No.1 | 0.7[28] | 5 | ||||
| Furan | 2 | 14 | IA | -36°C | ||
| Gasoline (100 Octane) | 1.4 | 7.6 | IB | < −40°C (−40°F) | 246 - 280°C | |
| Glycerol | 3 | 19 | 199°C | |||
| Heptane, n-Heptane | 1.05 | 6.7 | -4°C | 0.24 @ 3.4% | 204 - 215°C | |
| Hexane, n-Hexane | 1.1 | 7.5 | -22°C | 0.24 @ 3.8% | 225°C, 233°C[29] | |
| Hydrogen, dihydrogen, molecular H with two protons together | 4/17[30] | 75/56 | IA | Flammable gas | 0.016 @ 28% (in pure oxygen 0.0012) | 500 - 571°C |
| Hydrogen sulfide | 4.3 | 46 | IA | Flammable gas | 0.068 | |
| Isobutane | 1.8[31] | 9.6 | IA | Flammable gas | 462°C | |
| Isobutyl alcohol | 2 | 11 | 28°C | |||
| Isophorone | 1 | 4 | 84°C | |||
| Isopropyl alcohol, Isopropanol | 2[32] | 12 | IB | 12°C | 398 - 399°C; 425°C[33] | |
| Isopropyl chloride | IA | |||||
| Kerosene Jet A-1 | 0.6 - 0.7 | 4.9 - 5 | II | >38°C (100°F) as jet fuel | 210°C | |
| Lithium Hydride | IA | |||||
| 2-Mercaptoethanol | IIIA | |||||
| Methane (Natural Gas) | 4.4 - 5 | 15 - 17 | IA | Flammable gas | 0.21 @ 8.5% | 580°C |
| Methyl acetate | 3 | 16 | -10°C | |||
| Methyl Alcohol, Methanol | 6 - 6.7[34] | 36 | IB | 11°C | 385°C; 455°C[35] | |
| Methylamine | IA | 8°C | ||||
| Methyl Chloride | 10.7[36] | 17.4 | IA | -46 °C | ||
| Methyl ether | IA | −41 °C | ||||
| Methyl ethyl ether | IA | |||||
| Methyl ethyl ketone | 1.8[37] | 10 | IB | -6°C | 505 - 515°C[38] | |
| Methyl formate | IA | |||||
| Methyl mercaptan | 3.9 | 21.8 | IA | -53°C | ||
| Mineral spirits | 0.7[39] | 6.5 | 38-43°C | 258°C | ||
| Morpholine | 1.8 | 10.8 | IC | 31 - 37.7°C | 310°C | |
| Naphthalene | 0.9[40] | 5.9 | IIIA | 79 - 87 °C | ||
| Neohexane | 1.19[41] | 7.58 | −29 °C | 425°C | ||
| Nickel tetracarbonyl | 2 | 34 | 4 °C | 60 °C | ||
| Nitrobenzene | 2 | 9 | IIIA | 88°C | ||
| Nitromethane | 7.3 | 22.2 | 35°C | 379°C | ||
| Octane | 1 | 7 | 13°C | |||
| iso-Octane | 0.79 | 5.94 | ||||
| Pentane | 1.5 | 7.8 | IA | -40 to -49°C | as 2-Pentane 0.18 @ 4.4% | 260°C |
| n-Pentane | 1.4 | 7.8 | IA | 0.28 @ 3.3% | ||
| iso-Pentane | 1.32[42] | 9.16 | IA | 420°C | ||
| Phosphine | IA | |||||
| Propane | 2.1 | 9.5 - 10.1 | IA | Flammable gas | 0.25 @ 5.2% (in pure oxygen 0.0021) | 480°C |
| Propyl acetate | 2 | 8 | 13°C | |||
| Propylene | 2.0 | 11.1 | IA | -108°C | 0.28 | 458°C |
| Propylene Oxide | 2.3 | 36 | IA | |||
| Pyridine | 2 | 12 | 20 | |||
| Silane | 1.5[43] | 98 | IA | <21°C | ||
| Styrene | 1.1 | 6.1 | IB | 31 - 32.2°C | 490°C | |
| Tetrafluoroethylene | IA | |||||
| Tetrahydrofuran | 2 | 12 | IB | -14°C | 321°C | |
| Toluene | 1.2 -1.27 | 6.75 - 7.1 | IB | 4.4°C | 0.24 @ 4.1% | 480°C; 535°C[44] |
| Triethylborane | -20°C | -20°C | ||||
| Trimethylamine | IA | Flammable gas | ||||
| Trinitrobenzene | IA | |||||
| Turpentine | 0.8[45] | IC | 35°C | |||
| Vegetable oil | IIIB | 327°C (620°F) | ||||
| Vinyl acetate | 2.6 | 13.4 | −8 °C | |||
| Vinyl chloride | 3.6 | 33 | ||||
| Xylenes | 0.9 - 1.0 | 6.7 - 7.0 | IC | 27 - 32°C | 0.2 | |
| m-Xylene | 1.1[46] | 7 | IC | 25°C | 525°C | |
| o-Xylene | IC | 17 °C | ||||
| p-Xylene | 1.0 | 6.0 | IC | 27.2°C | 530°C |
See also
References
- ^ Britton, L. G “Using Material Data in Static Hazard Assessment.” as found in NFPA 77 - 2007 Appendix B
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ n-BUTYL MERCAPTAN ICSC: 0018
- ^ 2-HEXANONE ICSC:0489
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Cyanogen
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 211
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 216
- ^ CYCLOHEXANONE ICSC: 0425
- ^ MSDS Cyclopentadiene
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 221
- ^ CYCLOPENTANE ICSC: 0353
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 226
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 244
- ^ Walsh (1989) Chemical Safety Data Sheets, Roy. Soc. Chem., Cambridge.
- ^ Encyclopedia.airliquide.com
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 266
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 281
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 286
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 296
- ^ Yaws, Carl L.; Braker, William; Matheson Gas Data Book Published by McGraw-Hill Professional, 2001 pg. 301
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ http://environmentalchemistry.com/yogi/periodic/H.html
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Gases - Explosive and Flammability Concentration Limits
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
- ^ Combustibles
- ^ Working with modern hydrocarbon and oxygenated solvents: a guide to flammability American Chemistry Council Solvents Industry Group, pg. 7, January 2008
Further reading
- David R. Lide, Editor-in-Chief; CRC Handbook of Chemistry and Physics, 72nd edition; CRC Press; Boca Raton, Florida; 1991; ISBN 0-8493-0565-9